
6.1 - The Diffusion Chamber

HandsOn Activities:
22. The Diffusing Checkers Model
23. Deriving the Motion of Molecules
25. Location of the Precipitation Point
SimuLabs:
15. The Diffusion Chamber Simulation
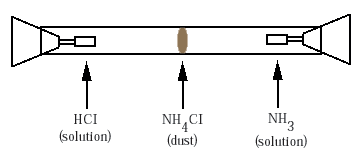
Ammonia gas is invisible as it moves through the air from Barry's opened bottle to Jennifer's nose. There is a simple demonstration that makes visible some consequences of this motion of ammonia. The demonstration uses what is called a diffusion chamber. This is a popular chemistry experiment in which two different gases, typically ammonia (NH3) and hydrogen chloride (HCl) diffuse from opposite ends of a closed glass tube (Figure ). Eventually the two gases meet and react, forming a disk of white dust made of the solid ammonium chloride (NH4Cl). We say that the ammonium chloride is precipitated out of the gas.
 |
Later you will be able to perform this experiment using small tubes supplied in your laboratory kit.
|